„GMP compliance services for ATMPs“
ATMPs
Our bioengineers, who specialize in cell and molecular biology, have an understanding of biopharmaceutical science, risk factors, and manufacturing processes related to cell and gene therapy, allowing us to provide input into the establishment of procedures in compliance with good manufacturing practices (GMP).
We contribute to scientific and laboratory assistance with genetic engineering, cell culture procedures and quality control operations. We supply hands-on dexterity with cell-based assays, vector engineering (AVV) and cell line engineering.
Currently there is a growing focus on gene therapy as research has made remarkable progress. New possibilities have emerged for the next generation of targeted treatments.
Consistent product quality is mandatory but especially for gene therapies arduous and demanding. Manufactures are in need of cost-effective processes to maximize productivity, purity, and yield and ensure efficacy, safety and process quality.
Compliant GMP manufacturing is hindered by small scale batches and labor-intensive processes. Cell and gene therapies require manufacturer to implement validated processes in order to guarantee safe handling of gene therapy.
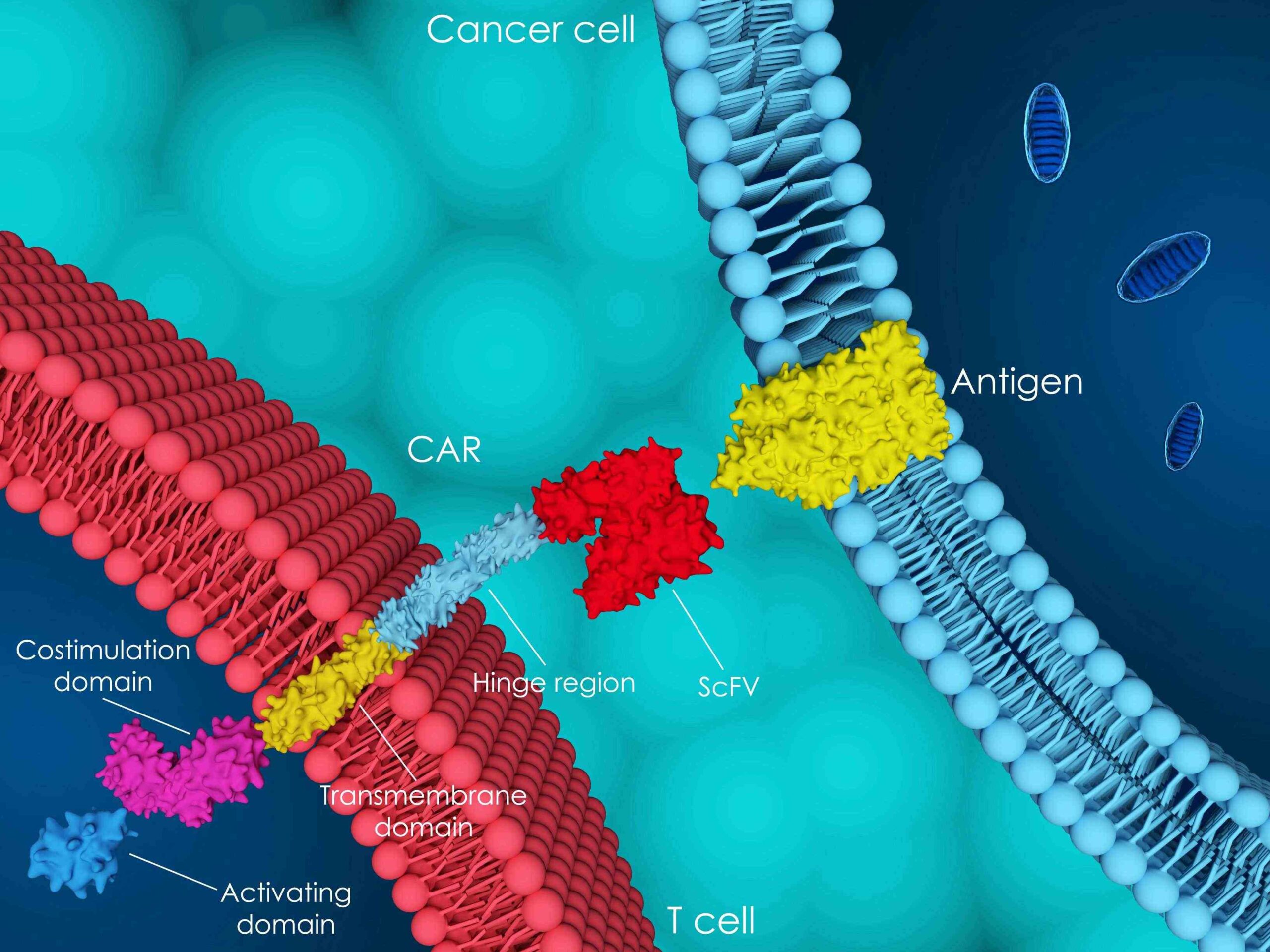
„We are contributing to strategies and validated operations for GMP release of gene and cell therapy.“
Our GMP Services for ATMPs
- Supporting scientific and laboratory assistance
- Facilitating the transition to GMP compliant processes

Your Benefits
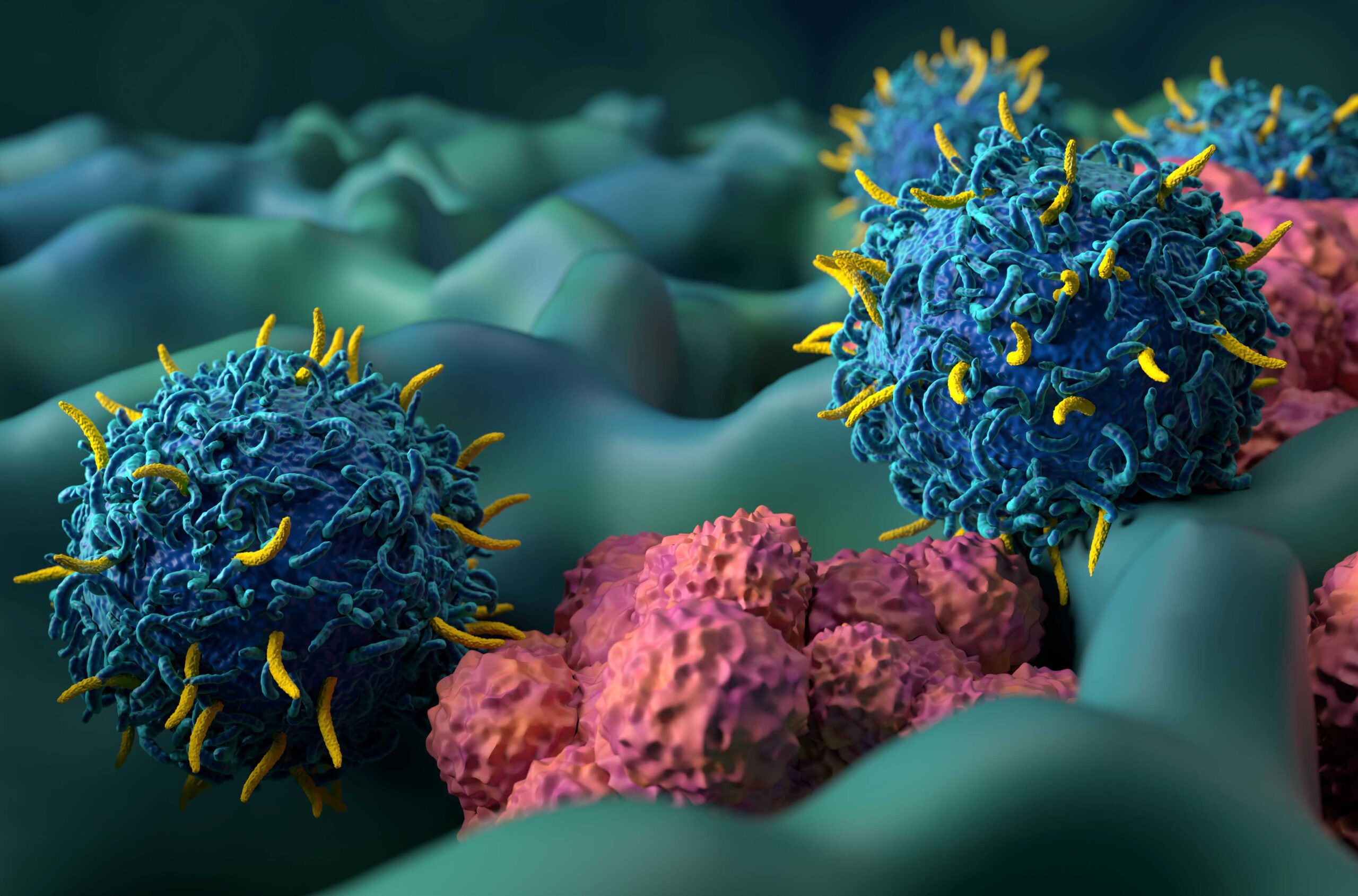
- GMP compliant manufacturing of cell and gene therapy (ATMPs)
- Manufacturing authorization
- Clinical trial and marketing authorization